Abstract
Background
AML in elderly patients (pts) is associated with poor outcomes and often arises from antecedent hematological disorders (AHD), classified as secondary AML (sAML). The primary objective of this study was to validate the use of somatic mutations to determine AML ontogeny in the elderly population (>70yo) in order to better distinguish de novo AML (pAML) versus sAML and their distinct outcomes.
Methods
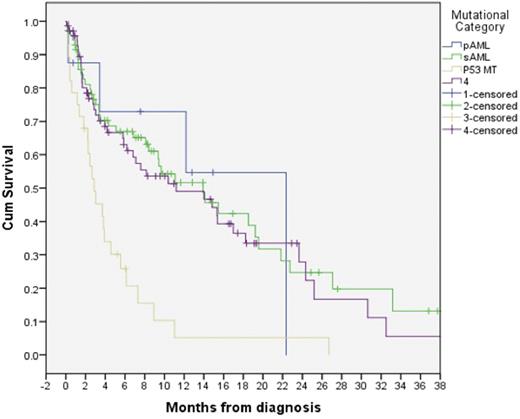
Utilizing the AML Database at Moffitt Cancer Center, we identified elderly pts with AML who had next generation sequencing (NGS) of up to 54 genes obtained at the time of diagnosis. Pts were divided into pAML or sAML based on prior history of AHD. Pts were then classified into 4 groups based on somatic mutation signature as suggested by Lindsley et al (Blood, 26 Feb 2015 x Vol125): group 1 (pAML) for pts with CBF rearrangements, 11q23/MLL gene rearrangements and NPM1 mutation (MT); group 2 (sAML) for those with SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 MT; group 3 for any pts who harbored TP53 MT; and group 4 as not otherwise specified (NOS).
Results
We identified 178 AML pts ≥ 70 years of age. The median overall survival (OS) was 9.4 months (mo). Based on clinical criteria, 95 pts were classified as pAML (no AHD) with a median OS of 11.2 mo and 82 pts were classified as sAML (prior AHD) with a median OS 5.1 mo (p=.21). Utilizing AML cytogenetic risk stratification only 4 pts met the criteria for good risk, 109 pts intermediate risk, and 57 pts poor risk. The median OS was 22.4,15.4, and 4 mo respectively (p<0.001).
Based on the molecular signature proposed by Lindsley et al, 8 pts were classified as pAML, 72 pts sAML, 28 pts had TP53 MT, and 70 pts were classified as NOS. The median OS was 22.4,14, 2.8, and 11.2 mo respectively for the 4 proposed groups based on molecular signature (p <0.001).
Clinical versus molecular classification was discordant where 25% of pts (n=2) classified as pAML by molecular signature had a history of AHD while 44% of pts (n=32) classified molecularly as sAML had no prior AHD. In TP53 MT and NOS categories, 37% (n=28) and 43% (n=70) of pts had AHD, respectively.
There was no difference in outcome based on first line therapy with hypomethylating agent (HMA) or intensive chemotherapy among any of the groups.
Conclusions
Molecular annotation of elderly AML patients by NGS reclassifies a significant proportion of patients as sAML. Pts molecularly categorized as sAML have worse outcomes than pts categorized as pAML, regardless of AHD history. Pts with TP53 MT are a distinct group who have particularly poor outcomes.
Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding. Sweet: Incyte: Research Funding; Otsuka: Consultancy; Pfizer: Consultancy; Karyopharm: Consultancy, Research Funding; Ariad: Consultancy, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Speakers Bureau. Lancet: Celgene: Consultancy; Erytech: Consultancy; Janssen: Consultancy; Jazz Pharmaceuticals: Consultancy; BioSight: Consultancy; Bio-Path Holdings: Consultancy; Boehringer Ingelheim: Consultancy; Novartis: Consultancy; Pfizer: Other: Institutional research funding, Research Funding. Komrokji: Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal